
Debunking Red Light Therapy Mechanisms: How Does it Really Work?
How much do we really know about the mechanisms of Red Light Therapy? Could the clinical science be misdirected towards trying to use the wrong explanation?
We know that Low Level Light Therapy (LLLT), photobiomodulation (PBM), AKA red light therapy has profound effects on improving mitochondria function, enhancing ATP production, modulating reactive oxygen species (ROS), and many more downstream effects. [1]
These fantastic effects are why it is so effective at helping nearly all parts of the body - from the skin all the way to the brain. But how is it really doing all of this?
The most prominent theory of red light therapy is the absorbance into Cytochrome C Oxidase (CCO), which is a protein and major component of the respiratory electron transport chain. Once provided this additional light energy, the CCO can dissociate from Nitric Oxide (NO) and up-regulate ATP production. The release of NO and ATP upregulation is primarily thought to be the initial starting point for all of the mechanisms and benefits. [1]
Nowadays, nearly all studies regurgitate this mechanism as part of the "background" or "introduction" section on the peer-reviewed studies on Pubmed. When Tina Karu had defined the action spectra and absorption spectrum of CCO this was considered a pivotal development in our understanding of Photobiomodulation. [2]

Figure 1. A common marketing gimmick is to show a generic LED spectrum overlaid with the CCO action spectra. Well here is the actual GembaRed Reboot overlaid with an approximate CCO action specta from [2]
But what if this is all wrong? Humans always try to oversimplify and need to explain things as part of our instinct to feel like we are in control. We have assigned individual gods to fundamental aspects of nature, found false correlations between saturated fats and heart disease, and even recently found that vitamin D (aka hormone D) is not the only reason why we need natural sunlight. Odds are pointing to that we probably don't have a solid grasp of the fundamentals of red light therapy mechanisms yet either.
As good scientists and engineers we need to be open to alternate view points, questioning our own knowledge and beliefs, and debating all the possibilities even if it seems trivial. If we fall into the "comfort zone" of belief and dogmatism, then that will actually stunt our potential for learning and growth.
In my viewpoint the CCO mechanism is too simplistic to explain all of the amazing benefits of red light therapy, which is what I explained in a podcast interview. Perhaps it is ONE of the mechanisms, or perhaps it is a very strong correlation that we are noticing.
The First Outlier
Sometimes it only takes one "outlier" to point you in a new direction. Some people want to throw away the outliers so you can have a nice, clean trend line. Or worse, some people hide the outliers because it would hurt their narrative (and sales). Good engineers know that we should embrace what we can learn from an outlier.
The first study that opened my eyes was one where they purposely tested PBM on cells that do not contain CCO! This remarkable study showed that they still had similar cell proliferation for cells lacking CCO and the controls.[3] Thus the conclusion that at least the cell proliferation aspects of PBM do not require CCO (or it's famous mechanisms).[3]
If this study doesn't open your eyes, then buckle up!
Isolated CCO Response:
Another recent study looked at isolated CCO and NO and exposed them to 670nm (Red) and 830nm (NIR) light. They found no significant activation in oxygen consumption from the CCO, nor any inhibition from the NO molecule. [9]
It seems some researchers are actively trying to disprove the CCO theory, for good reason if it is truly so flawed.
In these two studies we have:
1. Cells that show improvement without CCO.
2. Isolated CCO that shows no response to red or NIR light.
Is the Answer In Water Absorption?
In a peer-reviewed article titled "Aging Is A Sticky Business" the authors lay out a mechanism of mitochondrial aging where ROS accumulation in the mitochondria causes a slowing-down of the ATP production. The ROS causes an increase in viscosity (or, stickiness as implied by the title), and the absorption of NIR light can clear away the ROS and improve ATP production again.[4]
The EZ Water Connection
Although never directly cited by these alternative mechanism studies, this concept of light absorbing into water and reducing viscosity (stickiness) has been introduced by the work of Gerald Pollack and EZ Water, sometimes called structured water. [5]
The production of EZ Water has been found in Gerald Pollack's lab to form under two conditions:
1. Absorption of light into the water.
2. The water being near a hydrophilic surface (water-loving surface).
So no, you can't make EZ water by aiming your red light panel at a glass of water, because the glass isn't a hydrophilic surface. Dr. Pollack's lab typically uses Naphion tubing which is hydrophilic, but we also know inside of our cells and mitochondria have plenty of hydrophilic surfaces! [5]

Figure 2. Water has been known to support all life, perhaps it has a bigger role in photobiomodulation than we give it credit for.
When EZ water is formed it creates a "structure" of water that excludes or pushes away impurities. Thus leading to the potential removal of ROS or other molecules that increase the viscosity (stickiness). [5]
The Mitochondrial Interstitial Water Mechanism
In "Aging is a Sticky Business" the author argues that the probability of the light therapy reaching the CCO to perform the therapy is statistically very low. Whereas there is much more likelihood that there would be absorption into water near a hydrophilic surface.[4]
Another study proposing this mechanism discusses the nanoscopic interfacial water layer (IWL) which attaches to hydrophilic surfaces in the cells. They observe that Red and NIR lasers or LEDs will interact with the IWL by reducing the viscosity. Where if the viscosity was increased by ROS so much to become sticky and "glue-like", the modulation of IWL with Red/NIR light can remove the ROS and viscosity. [6]
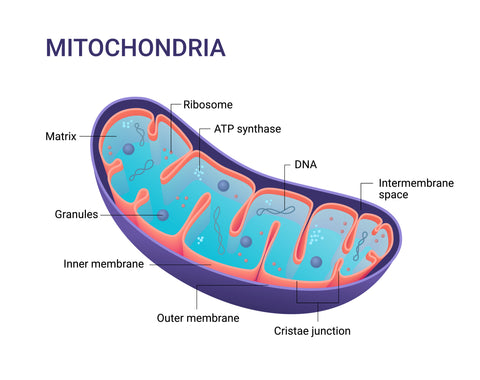
Figure 3. The surfaces inside and around the interstitial spaces in the mitochondria provide areas to form EZ water and promote this mechanism of action.
When the interstitial membranes of the mitochondria is "sticky" or glue-like from ROS, then that naturally will hinder the transfer of ATP and the mitochondrial rotary motor.[7] The decrease in viscosity of that IWL water caused by Red/NIR light is enough to restore that ATP rotor back to full function.
Water as a Well-Accepted PBM Chromophore
In the LLLT Handbook co-authored by the esteemed Dr. Hamblin, they have an entire chapter about how Water is a primary photoacceptor for photobiomodulation. [8]
Humans are 70% water by weight and 90% of our molecules are water! Statistically water is considered a huge target for PBM even though the Absorption Coefficient of these 600-1000nm wavelengths can be relatively low. [8]
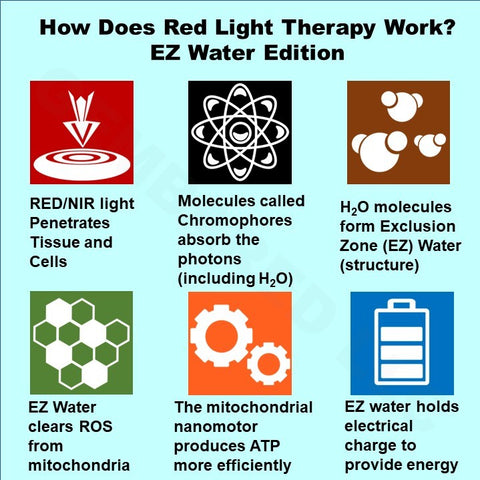
Within this chapter is a discussion on Interfacial Water and EZ Water! With EZ water comes changes in electrical and redox potential. Where it could be considered a bio-electric "battery" being charged by certain types of light absorption. [8]
So, we start to see how water-absorption is becoming an important consideration relevant to the mechanisms of PBM even in the mainstream PBM textbooks.
Conclusions
Although the CCO theory is trendy and exciting that photons can directly impact mitochondrial ATP production, this mechanism has been found to be implausible in several contexts. It is referred to as a "dogma" and "paradox" by some critics.
It might not be as "sexy" to accept the explanation of photobiomodulation is simply water-absorption. However, we can appreciate it's links to EZ-Water and the much more logical explanation presented by this alternate theory.
Figure 4. We know that red light therapy improves mitochondrial function, but we still need to work harder to understand HOW it works.
We previously wrote that instead of chasing THEORETICAL numbers like the action spectra of CCO, we used EMPIRICAL data from actual wavelengths that have proven to be useful in peer reviewed studies on Pubmed.
Like most things we write about in Red Light Therapy, it is complex and nuanced. There may not be one single explanation because it is multivariable and context dependent. We need to remain open to alternative and multiple mechanisms if we want to truly understand this amazing therapy.
[1]
Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516-533. doi:10.1007/s10439-011-0454-7
https://www.ncbi.nlm.nih.gov/
[2]
Karu, T. ACTION SPECTRA Their Importance for Low Level Light Therapy
Laboratory of Laser Biomedicine
Institute of Laser and Information Technologies
Russian Academy of Sciences
http://photobiology.info/Karu.html
[3]
Lima PLV, Pereira CV, Nissanka N, Arguello T, Gavini G, Maranduba CMDC, Diaz F, Moraes CT. Photobiomodulation enhancement of cell proliferation at 660 nm does not require cytochrome c oxidase. J Photochem Photobiol B. 2019 May;194:71-75. doi: 10.1016/j.jphotobiol.2019.03.015. Epub 2019 Mar 22. PMID: 30927704.
https://pubmed.ncbi.nlm.nih.gov/30927704/
[4]
Sommer AP. Aging Is a Sticky Business. Photomed Laser Surg. 2018 May;36(5):284-286. doi: 10.1089/pho.2017.4393. Epub 2018 Mar 23. PMID: 29570422.
https://pubmed.ncbi.nlm.nih.
[5]
Sharma A, Adams C, Cashdollar BD, et al. Effect of Health-Promoting Agents on Exclusion-Zone Size. Dose Response. 2018;16(3):1559325818796937. Published 2018 Sep 3. doi:10.1177/1559325818796937
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6122250/
[6]
- Published in Volume: 37 Issue 6: June 7, 2019
- Online Ahead of Print: May 20, 2019
https://www.liebertpub.com/
[7]
Sommer AP. Mitochondrial cytochrome c oxidase is not the primary acceptor for near infrared light-it is mitochondrial bound water: the principles of low-level light therapy. Ann Transl Med. 2019;7(Suppl 1):S13. doi:10.21037/atm.2019.01.43
https://www.ncbi.nlm.nih.gov/
[8]
Hamblin M, Pires de Sousa MV, Agrawal T. Handbook of Low-Level Laser Therapy 2017 Pan Stanford Publishing Pte. Ltd.
[9]
Quirk B, Whelan HT. Effect of Red-to-Near Infrared Light and a Nitric Oxide Donor on the Oxygen Consumption of Isolated Cytochrome c Oxidase. Photobiomodul Photomed Laser Surg. 2021 Jun 10. doi: 10.1089/photob.2020.4978. Epub ahead of print. PMID: 34115530.
https://pubmed.ncbi.nlm.nih.gov/34115530/